By Haojing Rong and Aimee Raleigh, as a part of the From The Trenches function of LifeSciVC
This weblog submit is the second in a collection on key diligence ideas and questions. When you missed the intro weblog submit yesterday, click on right here.
For this deeper take a look at pharmacology, we shall be specializing in a theoretical small molecule inhibitor and constructing out the totally different “clues” to search for as you pull collectively a PK/PD/efficacy relationship thesis. That stated, this submit is just scratching the floor of a really expansive house – for these fascinated about studying extra, we hyperlink to a handful of sources that could be useful. I’m delighted to show it over to Haojing Rong, SVP Translational Science at an Atlas Enterprise newco and a gifted DMPK scientist and chief with a powerful profession (former Kymera, Shire, Pfizer, Merck, Amgen, amongst others). Given her {qualifications}, she has graciously agreed to co-author this submit and share her views. With out additional ado, let’s dive in!
What’s pharmacology, and why is it simply as essential as biology for understanding a drug’s exercise? Typically instances, choosing the right mechanism with which to handle a (probably dysfunctional) goal in illness is just step one in growing a therapeutic. To realize the specified biology, we should have conviction that it (1) will get to the specified goal with ample exposures over a sure time period (pharmacokinetics/PK readout), (2) interacts with the goal within the desired trend, enacting proximal and distal “downstream” biology (pharmacodynamics/PD readout), and (3) displays pharmacological impact that impacts the illness in a approach to alter the medical course (efficacy readout). Every of those 3 readouts could be thought-about as a crucial piece of a puzzle – proof of 1 piece alone (e.g., efficacy in an animal mannequin) just isn’t ample by itself to justify shifting a program into the clinic, and a preclinical improvement staff’s mission is largely to find out if the puzzle items match collectively in a compelling story (Fig. 1).
Given the above 3 items of the broader pharmacology puzzle to resolve, what does a complete dataset appear like? Beneath we are going to define the steps to develop a PK/PD/efficacy relationship. For example all through this piece, we are going to use a small molecule inhibitor towards goal X for simplicity, although after all these ideas could be utilized extra broadly.
Pharmacokinetics (PK): Earlier than the drug does something to the physique (time period PD), the physique does quite a few issues to the drug (time period PK). These are Absorption, Distribution, Metabolism and Elimination, also called ADME. These processes decide the destiny of the drug (PK) in plasma and tissues all through the physique. The primary piece of the puzzle is to find out whether or not our small molecule will get to the location of curiosity (goal) at excessive sufficient concentrations to attain a desired exercise. To be able to do this, upon administration the small molecule should be absorbed, could must survive metabolism within the liver as first path, attain systemic circulation, and distribute to the tissue of curiosity (amongst others) all through the physique. Word that right here we discuss with exposures vs. dose – even when dose is held fixed, exposures will fluctuate by species and throughout compounds. For every compound, publicity on the web site of motion together with its efficiency ought to be thought-about when decoding the PD impact.
Simply as in actual property, PK is all about “timing” and “location”. The PK of our theoretical small molecule inhibitor shall be a time-concentration (publicity) profile and can fluctuate relying on route of administration (IV vs. PO vs. SC, and so on.). Many essential metrics, e.g., Cmax, Cmin, T1/2, and so on. could be derived from the PK profile to signify publicity at a given time and the way shortly it adjustments. That’s the “time” piece; what about “location”? That refers to tissues, which is the location of motion for many medication. Properties of the molecule, equivalent to lipophilicity, basicity, whether or not it’s a substrate of cell membrane transporters, and so on. typically end in totally different exposures in tissues vs. plasma. However, plasma PK is essentially the most helpful parameter when involves understanding in vivo PK/PD relationship. Subsequently tissue-to-plasma partition ratio, Okp, is a really helpful parameter to bridge essentially the most accessible PK (plasma) to essentially the most related PK (goal tissue).
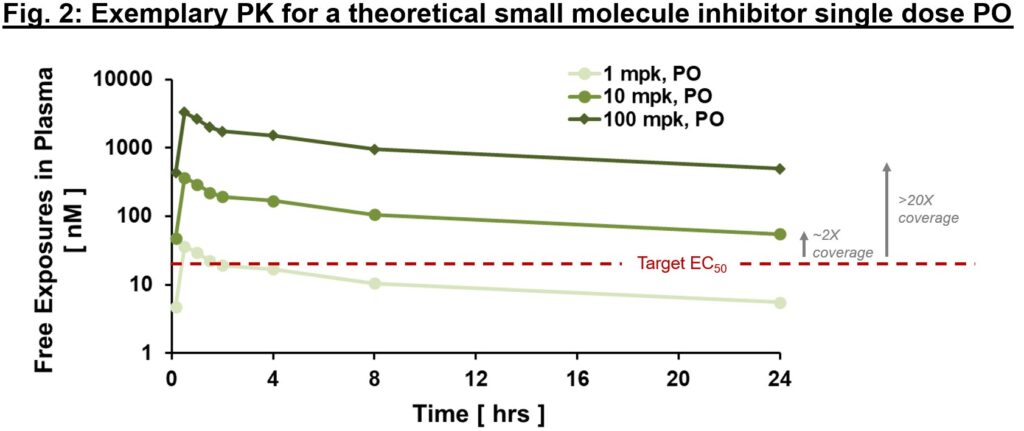
Typically you will need to distinguish between “whole” and “free” exposures, with the latter referring to the fraction of the small molecule that’s not protein-bound and thus is “free” to work together with the goal. An essential metric we frequently use to determine whether or not exposures are ample to exert downstream PD and efficacy is “multiples over EC50, EC90″”, and so on. the place EC50 is the compound’s half-maximal efficient focus. As in Fig. 2, free exposures plotted over EC50 (derived from an in vitro efficiency assay) might help directionally inform whether or not any “impact” you’re seeing in vivo is on-mechanism. With the belief of dose-proportional PK, one might ask the query of “what dose will present 2x vs. 20x EC50 protection at Cmin?” (Fig. 2). We expound beneath, however ideally PD and efficacy enhancements are commensurate with variations in publicity multiples above EC50 (at the very least as much as a sure level). If that’s the case, comparatively early on in drug improvement we are able to generate a thesis relating to goal protection required to enact a desired impact. In fact, that is one framework for evaluating potential impact of PK on PD or efficacy – there are situations the place EC50 is probably not ample. Greater multiples point out larger goal protection (e.g., EC90 or larger) is required for PD or efficacy. Though very fundamental, this framework is a robust diagnostic instrument (consider this as your annual bodily). If PK and efficiency multiples line up with PD and efficacy, an enormous congratulations to the mission staff! Not solely can one have cheap confidence within the thesis of this MoA, but in addition this means good IVIVc (in vitro–in vivo correlation). IVIVc is the inspiration of structure-activity relationship (SAR) for lead optimization, which is a course of to additional enhance an energetic compound to a secure and efficacious improvement candidate.
When fixing the primary piece of the PK puzzle, bear in mind “time” and “location (tissue of curiosity)”, use Okp (tissue to plasma partition ratio) to bridge plasma and tissue PK, deliver out the efficiency measurement EC50, and line all of them up for comparability. As soon as put collectively, these very fundamental data are surprisingly informative – an “aha” second from these relationships provides you with larger confidence within the validity of the mechanism and path ahead for acquiring a improvement candidate for medical testing. Conversely a “what’s happening?” response is commonly the start of a deepened understanding of the biology and pathway. Count on the “surprising” is a part of new ideation expertise. Turning “surprising” into “anticipated” is what brings all of us collectively on the journey of discovering new medicines.
Pharmacodynamics (PD): As soon as there’s a great deal with on the PK understanding, we search for “downstream” PD readouts reflecting proximal goal engagement and/or distal pharmacological exercise ensuing from the drug’s impact on the goal. Ideally a number of PD results could be leveraged; some could also be noisier than others, so finest to start out out with a number of PD readout choices and slender all the way down to these most related or constant. It’s important early within the drug discovery course of to establish PD readouts that may ideally be used throughout each early in vitro efficiency readouts all the best way to in vivo research in animals and people. Whereas not at all times possible, biomarkers that can be utilized throughout assays and mannequin techniques can enormously improve the power to generate IVIVc datasets to show or disprove the PK/PD/efficacy relationship. Leqvio (inclisiran, siRNA focusing on PCSK9) presents an excellent instance of PD markers, with goal engagement represented by decreased PCSK9 transcript and barely extra distal PD represented by decreased LDL ldl cholesterol. LDL-c is a circulating marker that may be measured precisely and with relative ease (and over each acute and persistent time programs) in mouse and nonhuman primate fashions, and is easy to judge in serum samples within the clinic for each wholesome volunteers and hypercholesterolemia sufferers. Different examples of well-supported PD readouts embrace circulating IgG for FcRn antagonists, pAKT for PI3K inhibitors, and serum tryptase for mast cell depletion methods.
Circling again to our publicity plot (Fig. 2), PD markers are anticipated to alter with publicity – for instance, at 1x vs. 3x vs. 10x multiples above EC50/IC50, one would anticipate progressively elevated PD impact (as much as a sure doubtless “saturating” publicity). If a PD readout just isn’t exposure-related (flat response and even inverse response), that could be an indication to re-evaluate the mechanistic speculation or the appropriateness of the chosen PD marker. There are various “shades” of PD biomarkers, goal engagement biomarkers, proximal or distal PD markers, and efficacy biomarkers. Some could be quantitative whereas some are merely qualitative. PD biomarkers are extra sturdy for some targets than others – e.g., for a lot of immune-oncology therapeutics there are a variety of oblique readouts for immune cell activation that, whereas not ample in isolation, mixed can function an inexpensive PD readout.
It takes a village to decide on PD biomarkers and use them to resolve the PD puzzle. It begins with couple of straightforward but key questions, “what can we anticipate to be taught from this biomarker with our drug?” and adopted by “what resolution we are going to make based mostly on this biomarker?” Sounds difficult, and it’s! On the similar time, biomarkers are crucial instruments in drug improvement to translate organic data to its software in human drugs. PD biomarkers are “the messengers” that inform us “what the drug is doing to the physique?” alongside the pathway, from goal interplay to pharmacology and finally efficacy. It’s our job to know what the PD biomarker will and won’t inform us, and to make use of it accordingly to information us on the journey of speculation testing. For an early-stage program (significantly with novel biology goal) in case your PD readout just isn’t correlated with exposures, take a second to develop an understanding of the disconnect and, if wanted, take into account various readouts.
Efficacy: Finally what we care about is commonly efficacy of a molecule, however efficacy alone just isn’t an alternative choice to pharmacology. If a compound is efficacious at a single excessive dose in an animal mannequin, it’s unattainable to disentangle whether or not that efficacy derives from on-target or off-target results (or, typically as is the case with small molecules, a mix). The PK and PD readouts mentioned above assist to set the stage for maximizing potential to attain efficacy in preclinical fashions, in addition to present a framework for decoding efficacy as a part of a broader optimization scheme for future molecules. Much like the PD readouts mentioned above, the best efficacy parameters in animal fashions relate carefully to major endpoints that can ultimately be utilized in medical trials, for instance tumor shrinkage, surrogate illness severity scores, ranges of circulating markers, weight reduction, and so on. Additionally it is excellent for the preclinical mannequin to be at the very least considerably predictive of human outcomes, although after all this excellent scenario gained’t apply for indications the place no precedent efficacious therapies exist. Sadly for some indications, the interpretation of mannequin techniques is a limitation, and whereas this function definitely shouldn’t discourage funding in areas of excessive unmet want however poor mannequin techniques, it does shift substantial de-risking inflection factors into the clinic.
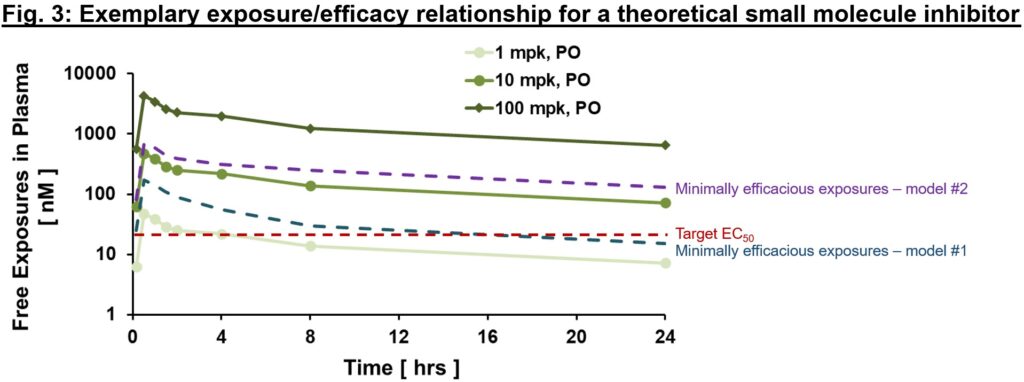
An exemplary publicity/efficacy mannequin is proven in Fig. 3, with exposures proven at steady-state (therefore they differ from Fig. 2 above given slight accumulation with time to steady-state values). Right here, we would assume that focus on protection (exposures ≥EC50) is required for at the very least 12 hrs a day (based mostly on findings from animal mannequin #1), and to be complete could plan for medical testing to cowl free exposures a lot larger than EC50 (≥100 nM, minimally efficacious in animal mannequin #2) to check the speculation with larger goal protection. Relying on the provision of a validated animal mannequin of human illness, totally different threat mitigation methods could also be thought-about. If a translational mannequin exists, the connection of publicityPD responseefficacy within the mannequin is invaluable to tell publicity required for efficacy and ample security window (also called therapeutic index, a subject for an additional day!) for the proof of idea (PoC) medical trial. Within the case {that a} mannequin doesn’t exist, PD biomarkers are our greatest wager to make sure that related pharmacology shall be examined within the clinic. That may permit us to check the medical PoC of latest goal, in a hypothesis-driven manner.
Remember the fact that we’re growing drugs for human illness. All preclinical fashions, together with human/patient-derived cell techniques and in vivo animal fashions, are instruments to information secure and environment friendly medical improvement. When engaged on novel targets for a illness with complicated biology (e.g. Alzheimer’s Illness) that contain a number of pathways relying on the stage and genetic disposition, related pharmacology fashions, whether or not in vitro or in vivo, are extra helpful to know publicity/PD relationship in vivo, which can be utilized to information dose and PD biomarker choice for a medical proof of idea (PoC) examine.
Now that we’ve mentioned the separate components of the PK/PD/efficacy relationship, how do these come collectively in preclinical improvement? Early in lead optimization, PK and PD are continuously assessed for a lot of compounds from totally different collection, and the early publicity/PD relationship might help (1) assist early theses on the right track protection required in addition to (2) additional refine optimization parameters as compounds proceed to be synthesized and examined. On the improvement candidate (DC) nomination stage, at the very least 1-2 DC contenders ought to have sturdy PK/PD/efficacy knowledge packages. Every of the three items of the pharmacology “puzzle” described above assist to tell how far-off we’re from the “end line” of our excellent base-case profile, and in doing so inform whether or not the preclinical improvement course of could also be a marathon or a dash.
With all this in thoughts, what are some widespread pitfalls in establishing PK/PD/efficacy relationships?
- Utilizing dose vs. exposures to check throughout species. PK finally is dependent upon a myriad of things (e.g., metabolism, which regularly varies barely by species) and thus exposures ought to at all times be used to benchmark molecules and to develop early assumptions for human efficacious dose vary. Evaluating doses throughout species or throughout molecules (“our competitor needed to dose at 100 mpk to attain tumor regression and we’re solely dosing at 50 mpk!”) is much less helpful on condition that dose (not like publicity) can’t be instantly in comparison with efficiency measures and used to extrapolate goal protection hypotheses.
- Utilizing efficacy as a “surrogate” for the PK/PD/efficacy relationship. Typically a novel hit is nominated in a screening assay early on and restricted funding is on the market for intensive chemistry synthesis and testing. Even so, it’s important to check for dose-ranging PK and PD forward of performing preclinical efficacy experiments. In our expertise in evaluating early seed firm pitches, it’s not unusual to see a single excessive dose examined in an animal mannequin to justify the complete thesis, however with out data of exposures and biomarkers that discriminate this efficacious dose from one that’s not. With out the complete knowledge bundle, it’s normally unattainable to find out whether or not efficacy is on-target (and biologically believable) and thus carries important threat in (pre-)medical testing.
- Testing too few compounds or solely shut analogues. Typically it’s useful to see the PK/PD/efficacy relationship maintain over disparate chemical matter, and thus testing compounds numerous in construction from a number of collection helps add confidence to the info bundle. Testing solely a single compound (virtually exceptional) or shut analogous could obscure elements of the connection. The extra range in molecules used to develop and take a look at the pharmacology speculation, the higher a speculation is de-risked earlier than going into human testing.
We hope this text serves as a useful introduction to a number of the datasets and relationships we frequently search for when evaluating a brand new goal, mechanism, thesis, and so on. in a brand new firm setting. That is, after all, a simplified description of a really complicated collection of subjects, and for readers who want to be taught extra, please try the beneath sources:
- “The Significance of PK-PD.” Barrow and Lindsley, Med. Chem. (2023).
- “Dose Predictions for Drug Design.” Maurer et al., Med. Chem. (2020).
- “Can the circulation of medicines be improved? Elementary pharmacokinetic and pharmacological ideas towards bettering Section II survival.” Morgan et al., Drug Discov At present (2012).
- “Software of Pharmacokinetic-Pharmacodynamic Modeling in Drug Supply: Growth and Challenges.” Zou et al, Entrance Pharmacol (2020).
- “A information to drug discovery” collection from Nature Critiques Drug Discovery (Jan 2023). https://www.nature.com/collections/hkgvrspwtn. A collection of articles focus on specific elements of the method of turning concepts into medication. Written by these carefully concerned within the discovery course of, these articles goal to supply insights that can support in future drug discovery applications.