By Cody Tranbarger, EIR at Atlas Enterprise, as a part of the From The Trenches characteristic of LifeSciVC
For many years, muscular dystrophy has stubbornly persevered among the many most intractable classes of human illness. Regardless of latest progress with AAV Gene Remedy and Antibody-Oligonucleotide Conjugates, a broad vary of muscular dystrophies are nonetheless untreatable, and collectively signify an infinite unmet medical want. On the core of those problems lies the Dystrophin-Glycoprotein Advanced (DGC) – a large, multi-protein construction accountable for anchoring muscle cells to their surrounding atmosphere. Whereas the DGC’s purposeful significance was understood as early because the Eighties, a unified image of its molecular structure has remained elusive, hampering efforts to develop actually focused therapies.
That’s, till now. Two latest research, printed in tandem by Nature (Liu et al. (2024), Wan et al. (2024)), have lastly unveiled near-atomic decision buildings of the native, totally intact DGC, providing profound insights that not each redefine our understanding of the advanced itself and open new therapeutic frontiers in muscular dystrophy. Collectively, they’re a tour de power of structural biology and, for my part, haven’t acquired almost the eye they deserve. On the threat of diving into the scientific weeds, what follows is an try to focus on the significance of those findings and their implications for drug growth in muscular dystrophy.
Wanting Again: Piecing Collectively a Patchwork DGC
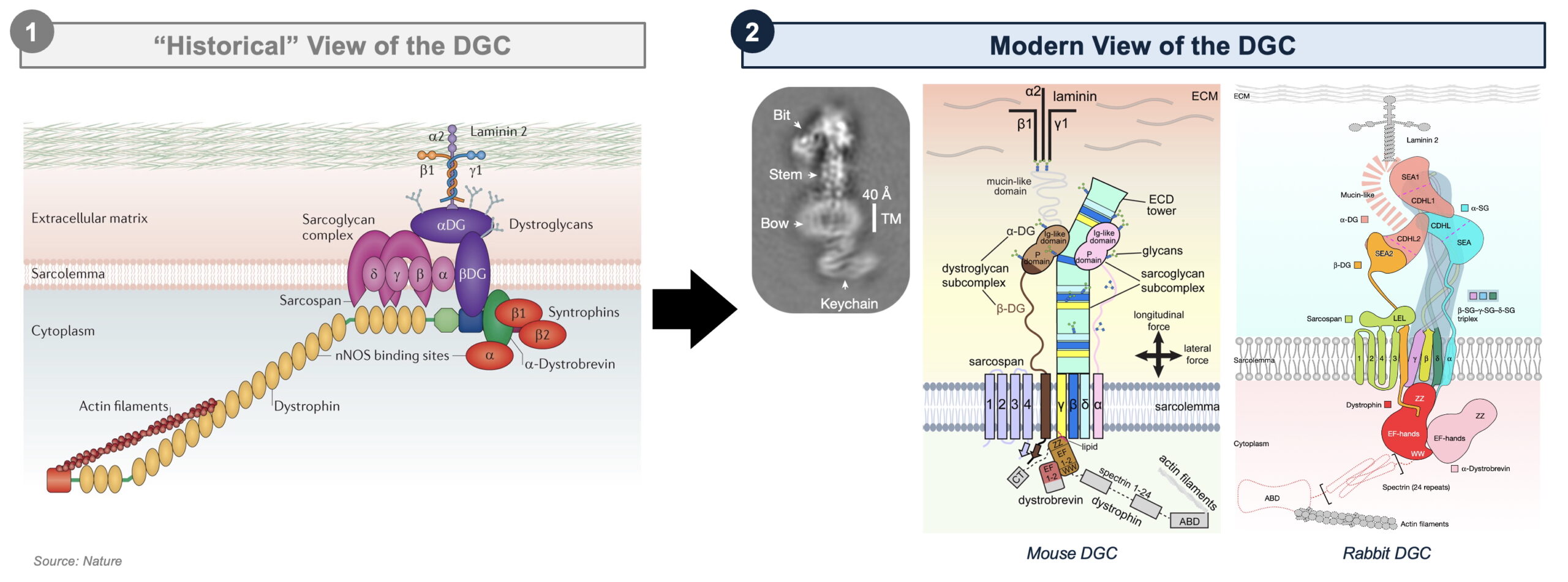
The DGC is a big, multi-protein meeting that performs a crucial position in sustaining muscle integrity by linking the intracellular actin cytoskeleton to the extracellular matrix (ECM). This structural connection acts as a molecular shock absorber, facilitating lateral and vertical power transmission whereas defending muscle fibers from contraction-induced injury. The DGC consists of three main subcomplexes (with frustratingly comparable names): the dystrophin-associated cytoskeletal advanced, which incorporates dystrophin and α-dystrobrevin that anchor actin to the membrane; the dystroglycan subcomplex, composed of α- and β-dystroglycan, which bridges dystrophin to ECM proteins like laminin-211; and the sarcoglycan-sarcospan subcomplex, containing α-, β-, γ-, and δ-sarcoglycans, in addition to sarcospan, which collectively stabilize the advanced inside the sarcolemma (muscle cell membrane). Genetic loss-of-function variants in lots of DGC parts compromise the mechanical stability of the sarcolemma, leading to numerous muscular dystrophies, equivalent to Duchenne Muscular Dystrophy (DMD), Limb-Girdle Muscular Dystrophy (LGMD), and Congenital Muscular Dystrophy (CMD). As such, the DGC and its underlying biophysical and mechanical properties have lengthy been of excessive curiosity for lecturers and drug builders alike.
Regardless of a long time of examine, a whole structural mannequin of the DGC remained out of attain. Early work all through the Nineties and early 2000s relied on co-immunoprecipitation, domain-specific crystallography, and nuclear magnetic resonance to investigate particular person DGC parts, equivalent to dystrophin’s WW and ZZ domains, dystroglycan’s ECM-binding glycosylation residues, particular person sarcoglycan subunits, and the cytoplasmic syntrophins and α-dystrobrevin. These strategies, nonetheless, had been incapable of capturing the total multi-protein meeting, resulting in a patchwork of assumptions which have till now comprised the sector’s unified view of the DGC, equivalent to:
- Dystroglycan has been assumed to be the core anchor of the DGC.
- Sarcoglycans have been thought to type an unbiased subcomplex on the periphery of the DGC.
- Sarcospan has been believed to play a minor, auxiliary position in stabilizing the sarcoglycan advanced.
- Dystrophin’s binding interactions have been solely partially deciphered, and the WW area’s binding to β-dystroglycan has been considered the dominant interplay.
This incomplete image of the DGC has constrained the event of latest therapeutic methods in muscular dystrophy. And not using a totally resolved construction, efforts to design focused interventions have been hampered by uncertainty relating to which domains are important for operate, how subunits work together dynamically, and the way disease-causing mutations disrupt DGC meeting. In consequence, most therapeutic mechanisms directed towards DGC restoration / stabilization, equivalent to utrophin and GALGT2, have failed; and even people who have succeeded in attending to FDA approval, equivalent to micro-dystrophin and exon skipping, have left a lot to be desired when it comes to precise purposeful advantages for sufferers.
Breaking By way of: Cryo-EM Cracks the DGC
Enter Cryo-Electron Microscopy (Cryo-EM). In contrast to conventional X-ray crystallography, which requires the protein of curiosity to type secure crystals, Cryo-EM permits the visualization of huge, versatile protein assemblies of their native states. Since its emergence within the early 2010s, Cryo-EM has revolutionized structural biology, enabling breakthroughs in our understanding of a broad vary of advanced macromolecular assemblies, together with the ribosome, the spliceosome, ion channels, viral capsids; and now, the DGC. Liu et al. and Wan et al. superbly combine single-particle Cryo-EM and computational modeling to realize the primary excessive decision 3D reconstruction of the intact DGC from mouse and rabbit skeletal muscle at a formidable 3.5–4.3 Å decision.
Collectively, these research provide a number of new revelations; among the many most important are:
- A brand new structural core: quite than their beforehand assumed peripheral location, β-, γ-, and δ-sarcoglycans type a central scaffold – an extracellular “tower” comprised of a triple β-helix – that stabilizes dystroglycan and dystrophin.
- A crucial, and precedented, curvature: on account of particular amino acid variations, this sarcoglycan scaffold assembles with a bent curvature, which can guarantee sarcolemma stability throughout contraction-induced stress. Notably, this characteristic has parallels to sure pathogens, which additionally make the most of bent β-helices to boost floor interactions and mechanical resilience, suggesting some extent of evolutionary conservation.
- A extra promiscuous Dystrophin: Slightly than binding solely to β-dystroglycan, dystrophin’s WW and ZZ area additionally work together with sarcoglycans, α-dystrobrevin, and lipids, revealing a extra advanced internet of interactions that regulate DGC integrity.
- A revised position for Sarcospan: Slightly than instantly binding α-dystroglycan, sarcospan stabilizes β-dystroglycan by positioning it between sarcoglycans, clarifying its position as a structural anchor inside the sarcolemma.
Not solely does this work rewrite the textbook mannequin of DGC meeting, but it surely additionally deepens our understanding of the molecular pathology of muscular dystrophy. Between the 2 research, greater than 110 pathogenic mutations had been structurally rationalized, instantly linking single-residue modifications to DGC destabilization, and establishing a directional genotype-phenotype relationship between the extent of DGC dysfunction and illness severity throughout quite a lot of muscular dystrophies pushed by loss-of-function in particular person DGC parts.
Wanting Ahead: From Constructions to Medicines
The structural decision of the DGC represents a milestone a long time within the making; and these structural advances carry essential implications, each for present mechanisms and for future therapeutic avenues.
First, they supply steerage for the design and refinement of micro-dystrophin gene remedy constructs for DMD. By necessity, micro-dystrophins lack massive areas of the wild-type protein, and there was substantial debate over time relating to optimum assemble design. Distinguished amongst these controversies has been the significance of retaining sure domains, such because the C-terminal coiled-coil area that was believed to mediate binding to α-dystrobrevin. These buildings, nonetheless, suggest and validate a newly found binding interplay between dystrophin’s WW area and α-dystrobrevin, seemingly refuting the necessity for coiled-coil inclusion and additional reinforcing the indispensability of the WW area.
These findings additionally present a roadmap for the event of sarcoglycan-directed small molecule correctors for LGMDs. Because the authors methodically display, many LGMD missense mutations drive pathology by destabilizing the β-helix ECD tower. Due to this fact, if a small molecule chaperone might obtain partial correction of sarcoglycan misfolding and / or localization, it might restore DGC meeting and stability. Cystic Fibrosis is the apparent parallel, and actually, CFTR correctors have demonstrated the flexibility to rescue LGMD2D on account of α-sarcoglycan (SGCA) missense variants in vitro and in vivo – a promising start line.
Lastly, these new learnings permit us to look past the DGC with higher confidence by offering worthwhile insights into the molecular interfaces between particular person DGC parts and their ECM ligands. This newfound visibility permits for deeper interrogation of the dysfunctional DGC / ECM interactions that underlie a number of underserved subtypes of LGMD and CMD, and should open up new therapeutic alternatives for focused organic approaches within the extracellular house.
Whereas loads of translational challenges stay, these research have armed the sector with a molecular framework to information next-generation drug discovery efforts. If historical past is any indicator, a brand new wave of therapies could also be on the horizon.
Right here at Atlas Enterprise, we’re considering deeply about all these new avenues and extra. We’re proud to have performed a task in advancing innovation for musculoskeletal problems at Dyne and elsewhere, and we’re excited to proceed constructing nice corporations that may convey nice medication to muscular dystrophy sufferers worldwide. Keep tuned.
References
Liu, Shiheng et al. “Native DGC construction rationalizes muscular dystrophy-causing mutations.” Nature vol. 637,8048 (2025): 1261-1271. doi:10.1038/s41586-024-08324-w
Wan, Li et al. “Construction and meeting of the dystrophin glycoprotein advanced.” Nature vol. 637,8048 (2025): 1252-1260. doi:10.1038/s41586-024-08310-2